Asymmetric VSD closure device
Asymmetric VSD closure device
An asymmetric VSD closure device has a left ventricular disc with a narrower flange towards the aortic side to reduce the chance of impinging on the aortic valve. Amplatzer asymmetric ventricular septal defect occluder is one type [1]. The left ventricular disc is typically 5 mm towards the left ventricular apex while it is only 0.5 mm towards the aorta.
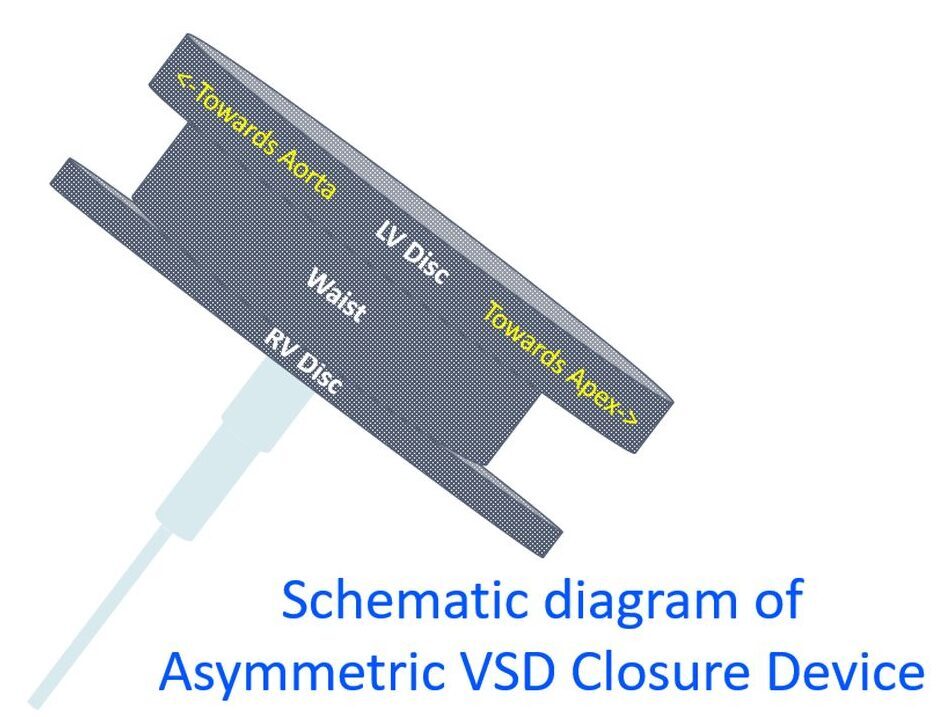
The connecting waist is 1.5 mm long and the right ventricular disc is 4 mm larger than the waist. The asymmetric VSD closure device is made of nitinol mesh covered with dacron fabric to facilitate thrombogenesis so that the final occlusion of the defect is by thrombus formation in the mesh [2].
Complete heart block is an important risk of device closure of perimembranous ventricular septal defect. This is because of pressure from the device damaging the atrioventricular conduction system which passes along the posterior border of the VSD. Other possibilities are trauma during the implant procedure leading to local hematoma formation and inflammatory response to the material of the device.
A study published in 2021 had used asymmetric VSD closure devices in 16 cases of perimembranous VSD without aortic margin. Three such devices were implanted in those with thick aortic margin as well. Three patients with associated membranous septal aneurysm were also closed with asymmetric VSD closure device. There were no late aortic regurgitation or heart block in that study on follow-up exceeding 7 years [3].
Potential complications of device closure of VSD
These include device migration, pericardial effusion, embolic episodes, delayed onset of conduction disturbances or even infective endocarditis. Fortunately, these complications are rare.
As with other intracardiac device closures, subjects are started on aspirin to prevent thrombus formation on the device, which is continued for six months which is the period required for endothelialization of the device surface.
References
- Pinto RJ, Dalvi BV, Sharma S. Transcatheter closure of perimembranous ventricular septal defects using amplatzer asymmetric ventricular septal defect occluder: preliminary experience with 18-month follow up. Catheter Cardiovasc Interv. 2006;68:145-52.
- Thanopoulos BD, Tsaousis GS, Karanasios E, Eleftherakis NG, Paphitis C. Transcatheter closure of perimembranous ventricular septal defects with the Amplatzer asymmetric ventricular septal defect occluder: preliminary experience in children. Heart. 2003; 89: 918–922.
- Singhi AK, Sivakumar K. Echocardiographic Classification of Perimembranous Ventricular Septal Defect Guides Selection of the Occluder Design for Their Transcatheter Device Closure. J Cardiovasc Imaging. 2021 Oct;29(4):316-326. doi: 10.4250/jcvi.2020.0218. Epub 2021 Mar 30. PMID: 34080335; PMCID: PMC8592680.