Phrenic nerve stimulation for central sleep apnea
Phrenic nerve stimulation for central sleep apnea
Central sleep apnea (CSA) is due to lack of respiratory drive during sleep causing inadequate ventilation. A rare congenital form known as congenital central hypoventilation syndrome (CCHS) has been called Ondine’s curse. Though central sleep apnea is rare, it is frequently associated with heart failure, atrial fibrillation and stroke [1].
The remedé system (Respicardia Inc, Minnetonka, MN, USA) is an implantable phrenic nerve stimulation system. Transvenous phrenic nerve stimulation causes diaphragmatic contraction similar to normal breathing. The remedé System Pivotal Trial randomized 151 patients across three countries in 31 hospitals [2]. Patients had apnea hypopnea index (AHI) of at least 20 events per hour as documented by polysomnography. All patients were implanted with device and randomly assigned in 1:1 ratio to either stimulation or no stimulation for a period of 6 months. 35 patients assigned to stimulation had a reduction of AHI of 50% or more at 6 months. The corresponding number in the control group was 8 (p<0·0001). There was concordant improvement in oxygenation and quality of life. Seven patients died, unrelated to implant system or therapy. Two each occurred in the treatment and control groups during the 6 month randomization period. Three deaths occurred between 6 months to 12 months period when all patients received neurostimulation. 27 patients in the treatment group had non-serious therapy related discomfort of which 26 had resolution on simple system reprogramming while one was unresolved. The device was approved by FDA in 2017.
A prior pilot study in 57 patients was reported in 2015 [2]. The remedē pulse generator is similar in size and appearance to a standard pacemaker. It is implanted in the right or left pectoral region. It uses a transvenous lead implanted in either the left pericardiophrenic or right brachiocephalic vein to provide stimulation to the adjacent phrenic nerve. Respiration is sensed by either the stimulation lead or another lead implanted in the azygous vein. This pilot study achieved its primary end point demonstrating 55% reduction in apnea-hypopnea index from baseline at 3 months.
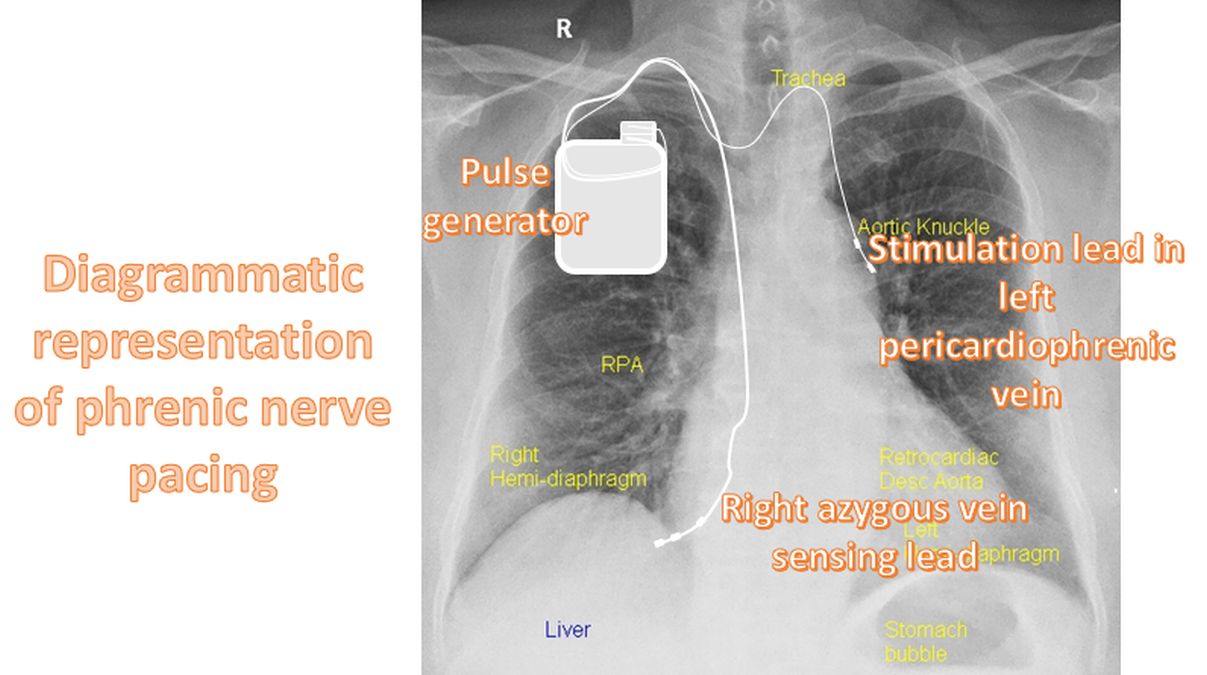
Five year follow up of the remedé System Pivotal Trial mandated by FDA was published in 2021 [1]. 52 of the original 151 patients completed the five year follow up. The study documented sustained improvement in apnea-hypopnea index, central-apnea index, arousal index, oxygen desaturation index and sleep architecture. The Epworth Sleepiness Scale (ESS) had a median reduction of 3 points at 5 years, which was statistically significant. 16 patients underwent pulse generator reposition or lead revision, primarily in the first year. No unanticipated adverse device effects or related deaths occurred through out the 5 year period.
A substudy evaluated the interaction with concomitant cardiovascular implantable electronic devices (CIEDs) [4]. 64 of the 151 patients had concomitant CIED. Four CIED oversensing events were noted in 3 patients which lead to one inappropriate defibrillator shock and delivery of antitachycardia pacing. The presence of CIED did not seem to impact implant metrics, implantation success and efficacy of transvenous phrenic nerve pacing. They suggested following a detailed interaction protocol to minimize the occurrence of device-device interaction.
References
- Costanzo MR, Javaheri S, Ponikowski P, Oldenburg O, Augostini R, Goldberg LR, Stellbrink C, Fox H, Schwartz AR, Gupta S, McKane S, Meyer TE, Abraham WT; remedē®System Pivotal Trial Study Group. Transvenous Phrenic Nerve Stimulation for Treatment of Central Sleep Apnea: Five-Year Safety and Efficacy Outcomes. Nat Sci Sleep. 2021 Apr 29;13:515-526. doi: 10.2147/NSS.S300713. PMID: 33953626; PMCID: PMC8092633.
- Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, Abraham WT; remedé System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016 Sep 3;388(10048):974-82. doi: 10.1016/S0140-6736(16)30961-8. Epub 2016 Sep 1. PMID: 27598679.
- Abraham WT, Jagielski D, Oldenburg O, Augostini R, Krueger S, Kolodziej A, Gutleben KJ, Khayat R, Merliss A, Harsch MR, Holcomb RG, Javaheri S, Ponikowski P; remedē Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail. 2015 May;3(5):360-369. doi: 10.1016/j.jchf.2014.12.013. Epub 2015 Mar 11. PMID: 25770408.
- Nayak HM, Patel R, McKane S, James KJ, Meyer TE, Germany RE, Stellbrink C, Costanzo MR, Augostini R. Transvenous phrenic nerve stimulation for central sleep apnea is safe and effective in patients with concomitant cardiac devices. Heart Rhythm. 2020 Dec;17(12):2029-2036. doi: 10.1016/j.hrthm.2020.06.023. Epub 2020 Jun 30. PMID: 32619739.

