Ductus Dependent Circulation
Ductus Dependent Circulation
Ductus dependent circulation is one in which a patent ductus arteriosus is useful in maintaining the circulation after birth. It is important to rule out such conditions before any PDA closure is planned. PDA dependent circulations can be PDA dependent pulmonary circulation, PDA dependent systemic circulation and PDA dependent mixed circulation. The first group includes pulmonary atresia, tricuspid atresia and tetralogy of Fallot with pulmonary atresia. In those cases, post natal physiological constriction of ductus arteriosus can cause severe hypoxemia, cyanosis and even death. In these cases, there is severe restriction of pulmonary blood flow and pulmonary circulation is dependent on a PDA [1].
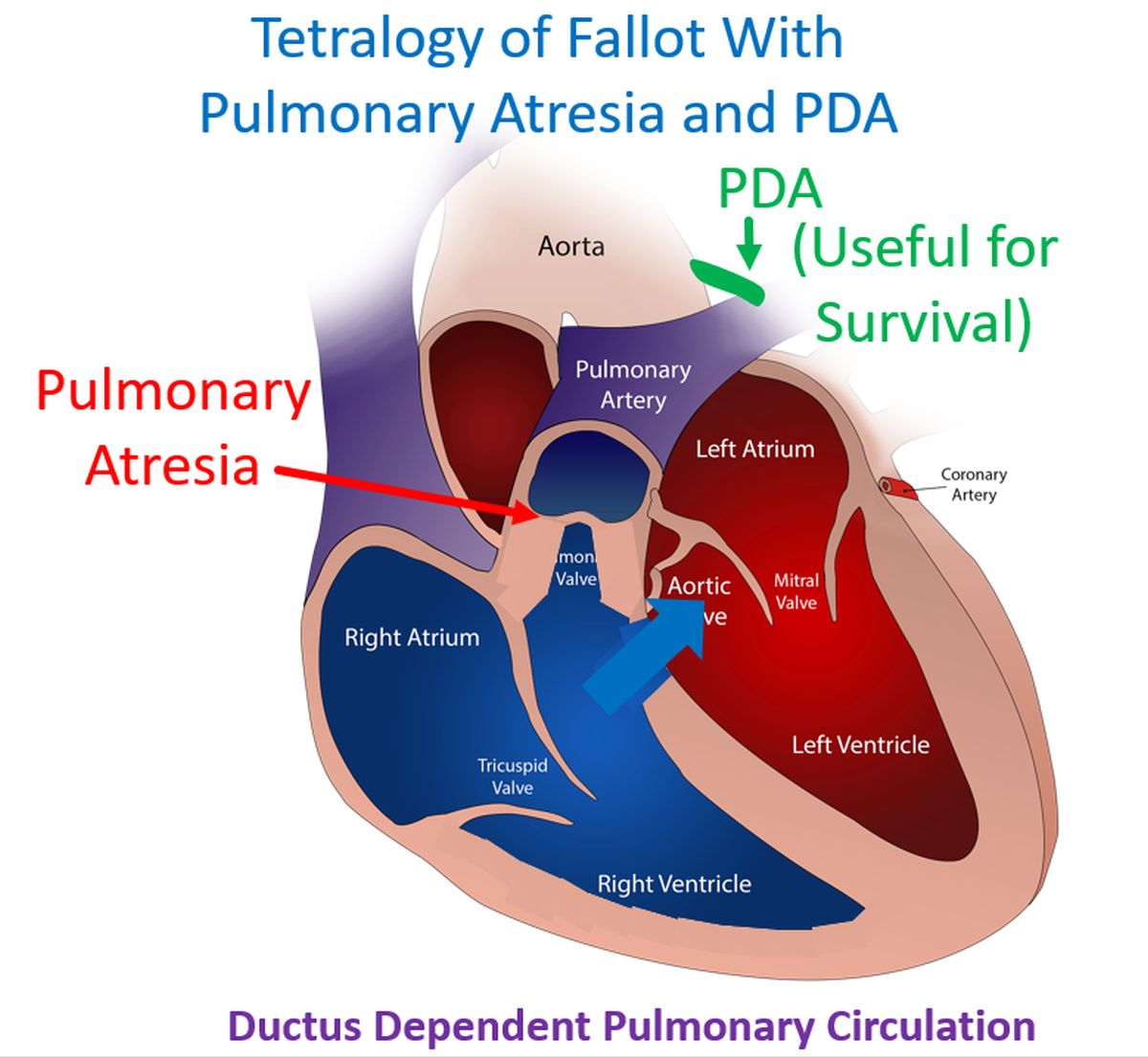
Second group of conditions with severe restriction of systemic blood flow include severe aortic stenosis, coarctation of aorta, interrupted aortic arch and hypoplastic left heart syndrome. Postnatal constriction of PDA can cause systemic hypoperfusion, shock, metabolic acidosis and death. In the third group as in transposition of great arteries, both systemic and pulmonary circulations are dependent on adquate mixing as both circulations are in series.
Maintaining a PDA is one method of providing mixing between systemic and pulmonary circulations. In all these situations, ductal patency can be maintained by prostaglandin E1 infusion as a temporary measure till other procedures can be done. Continuous infusion is needed as 60-80% of PGE1 is metabolized on first through the lungs [1]. Initial palliation in transposition of great arteries can be with balloon atrial septostomy followed by arterial switch operation at the earliest.
Two main ways of managing those with other varieties of ductus dependent circulation after the initial prostaglandin E1 infusion, are stenting the patent ductus arteriosus and modified Blalock-Taussig shunt. Multicentre trials have shown equal mortality endpoints for both methods, but reduced moribidity for ductal stenting than modified BT shunt. Recovery times are shorter and requirement for extracorporeal membrane oxygenation (ECMO) is lower with ductal stenting. There is also improved quality of life with ductal stenting. But the main limiting factor is the longevity of the stent [2].
A systematic review and meta-analysis comparing PDA stenting and surgical aortopulmonary shunts concluded that both had similar mortality. But there was shorter hospital stay, shorter duration of mechanical ventilation and fewer complications with PDA stenting. They found more patients with pulmonary atresia and intact ventricular septum with expected biventricular repair undergoing PDA stenting [3].
References
- Cucerea M, Simon M, Moldovan E, Ungureanu M, Marian R, Suciu L. Congenital Heart Disease Requiring Maintenance of Ductus Arteriosus in Critically Ill Newborns Admitted at a Tertiary Neonatal Intensive Care Unit. J Crit Care Med (Targu Mures). 2016 Nov 8;2(4):185-191. doi: 10.1515/jccm-2016-0031. PMID: 29967858; PMCID: PMC5953253.
- Zaidi M, Sorathia N, Abbasi H, Khashkhusha A, Harky A. Interventions on patent ductus arteriosus and its impact on congenital heart disease. Cardiol Young. 2020 Nov;30(11):1566-1571. doi: 10.1017/S1047951120004126. Epub 2020 Nov 23. PMID: 33222711.
- Tseng SY, Truong VT, Peck D, Kandi S, Brayer S, Jason DP 3rd, Mazur W, Hill GD, Ashfaq A, Goldstein BH, Alsaied T. Patent Ductus Arteriosus Stent Versus Surgical Aortopulmonary Shunt for Initial Palliation of Cyanotic Congenital Heart Disease with Ductal-Dependent Pulmonary Blood Flow: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2022 Jul 5;11(13):e024721. doi: 10.1161/JAHA.121.024721. Epub 2022 Jun 29. PMID: 35766251; PMCID: PMC9333373.
