Extravascular ICD Pivotal Study – Clinical Trial Review
Extravascular ICD Pivotal Study- Clinical Trial Review
എക്സ്ട്രാവാസ്കുലർ ഐസിഡി – പുതിയ മെഡിക്കൽ ഉപകരണം
Extravascular implantable cardioverter defibrillator is a novel concept. Lead systems are considered to be the Achilles heel of conventional transvenous ICDs. To overcome this subcutaneous ICDs were developed, to avoid the vascular risks of transvenous ICDs. But they were not capable of overdrive pacing for ventricular tachycardia [1]. The lead of subcutaneous ICD is placed between the sternum and skin so that larger current is needed for pacing and defibrillation. This would mean a larger device with higher battery capacity. The extravascular ICD uses substernal electrodes which avoids the sternal bone between the electrode and the myocardium as in the case of subcutaneous ICD [2].
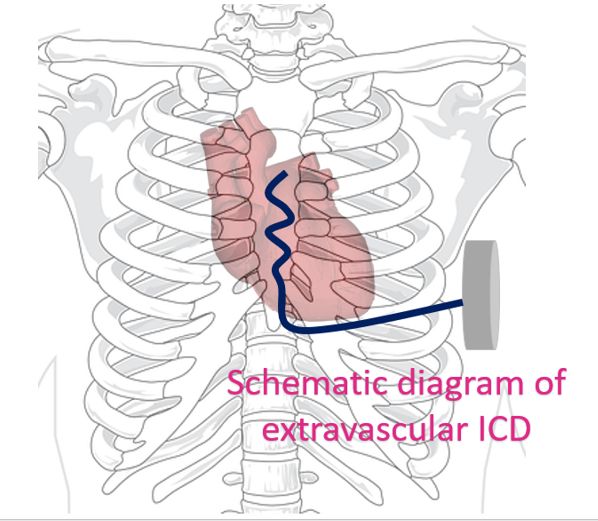
Extravascular ICD Pivotal Study was a prospective non-randomized premarket global clinical study involving patients with class I or IIa indication for an ICD [2]. The primary efficacy endpoint was successful defibrillation at implantation. Implantation was attempted in 316 patients of which 302 had induction of ventricular arrhythmia and defibrillation testing protocol completed. Success of defibrillation was 98.7%. 299 of the 316 patients were discharged with a working ICD system. Freedom from major system or procedure related complications at 6 months was 92.6%.
The success rate of antitachycardia pacing was 50.8%. A total of 29 patients received 118 inappropriate shocks for 81 arrhythmic episodes. Eight systems were explanted without extravascular ICD replacement over a mean follow up period of 10.6 months. The pulse generator of an extravascular ICD is implanted in the left midaxillary line and can deliver shocks up to 40 Joules. The procedure was done either in cardiac catherization laboratory or hybrid operating room by cardiologists who underwent a structured hands-on training program.
The advantage of extravascular ICD over subcutaneous ICD is that proximity of the lead to the myocardium permits successful antibradycardia and antitachycardia pacing. The median energy for defibrillation was 15 Joules at implantation, which was similar to that of transvenous ICDs and about half of that reported with subcutaneous ICDs [3]. A caveat is that implantation of the lead in substernal space requires special training in collaboration with cardiac surgeon as substernal space is not conventionally accessed by cardiologists.
An interesting feature was that most common reason for inappropriate shocks in extravascular ICD was P wave oversensing, which was explained by the proximity of the substernal lead to the right atrial appendage. No cases of mediastinitis, sepsis or endocarditis related to the extravascular ICD were noted in the current study. Infections had occurred in the pulse generator pockets. Four of the 8 removals of extravascular ICDs were due to infection.
We need data on more patients before we can conclude that the smaller device size of extravascular ICD compared to subcutaneous ICD translates to lesser pocket hematoma and pocket infection rates. The novel concept of extravascular ICD looks promising. Of course, only time will tell whether it will actually replace subcutaneous and transvenous ICDs in future.
References
- Gold MR, Lambiase PD, El-Chami MF, Knops RE, Aasbo JD, Bongiorni MG, Russo AM, Deharo JC, Burke MC, Dinerman J, Barr CS, Shaik N, Carter N, Stoltz T, Stein KM, Brisben AJ, Boersma LVA; UNTOUCHED Investigators*. Primary Results From the Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction (UNTOUCHED) Trial. Circulation. 2021 Jan 5;143(1):7-17. doi: 10.1161/CIRCULATIONAHA.120.048728. Epub 2020 Oct 19. PMID: 33073614; PMCID: PMC7752215.
- Friedman P, Murgatroyd F, Boersma LVA, Manlucu J, O’Donnell D, Knight BP, Clémenty N, Leclercq C, Amin A, Merkely BP, Birgersdotter-Green UM, Chan JYS, Biffi M, Knops RE, Engel G, Muñoz Carvajal I, Epstein LM, Sagi V, Johansen JB, Sterliński M, Steinwender C, Hounshell T, Abben R, Thompson AE, Wiggenhorn C, Willey S, Crozier I; Extravascular ICD Pivotal Study Investigators. Efficacy and Safety of an Extravascular Implantable Cardioverter-Defibrillator. N Engl J Med. 2022 Aug 28. doi: 10.1056/NEJMoa2206485. Epub ahead of print. PMID: 36036522.
- Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, Fynn SP, Murgatroyd FD, Sperzel J, Neuzner J, Spitzer SG, Ardashev AV, Oduro A, Boersma L, Maass AH, Van Gelder IC, Wilde AA, van Dessel PF, Knops RE, Barr CS, Lupo P, Cappato R, Grace AA. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010 Jul 1;363(1):36-44. doi: 10.1056/NEJMoa0909545. Epub 2010 May 12. PMID: 20463331.

