How good is a wearable cardioverter defibrillator?
How good is a wearable cardioverter defibrillator?
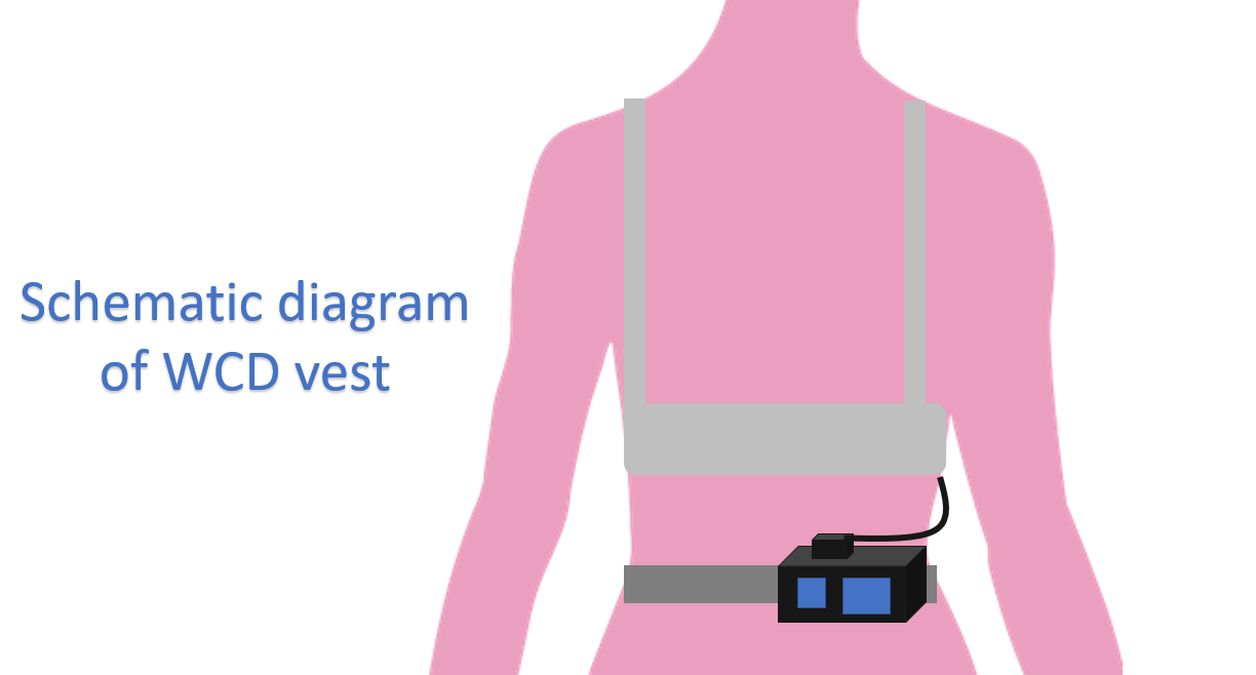
Unlike the implantable cardioverter defibrillator (ICD) which is implanted subcutaneously, wearable defibrillator (WCD) is worn as a vest. Defibrillation patch electrodes and monitoring system are included in the vest and the defibrillator with battery is kept on the belt externally. WCD automatically detects and treats ventricular tachyarrhythmias without the need for assistance from bystander. Though the original device used monophasic shocks, later ones had a biphasic shock waveform [1].
The biphasic device had a maximum output of 150 Joules and could terminate ventricular fibrillation at the first attempt with 70 Joules in 12 and 100 Joules in 10 of the episodes tested. Still programming at maximum energy output has been suggested for ambulatory patients to ensure good safety margin. WCD has been called as a “Life vest till the life boat arrives”, meaning that it can be considered during the initial window period after acute myocardial infarction when the patient is not eligible for an ICD as per guidelines [2].
One study of real-world utilization of WCD was reported in 2017. Of the total 140 patients, 46% had nonischemic cardiomyopathy and 32% had ischemic cardiomyopathy. Some of the patients had genetic predisposition for sudden cardiac death while some had an ICD explanted. Mean daily use of WCD was for 17.3 ± 7.5 hours. The usage time was lower than the 21.3 hours presented by Dillon KA et al in another study [3-5]. Usage time implies an Achilles heel of WCD, compliance! The lower the usage time, more likely that an unprotected arrhythmic event can occur.
A systematic review and meta-analysis published in 2019 found 28 studies of which only one was a randomized control trial while 27 were observational studies. There were 32,426 patients in the studies reported [6]. Appropriate WCD therapy occurred in 5 per 100 persons over 3 months. In the VEST trial [7], the incidence was 1 per 100 person years over 3 months, compared to 11 per 100 persons over 3 months in the observational studies. Inappropriate WCD therapy occurred at a rate of 2 per 100 persons over 3 months. Mortality while wearing WCD was rare, 0.7 per 100 persons over 3 months.
VEST (Vest Prevention of Early Sudden Death) trial had randomly assigned in a 2:1 ratio, patients with acute myocardial infarction and an ejection fraction of 35% or less to receive a WCD with guideline directed medical therapy or guideline directed medical therapy alone [7]. Of the total 2302 patients, 1524 were allotted to WCD and 778 to control group. Arrhythmic death occurred in 1.6% of the WCD group and 2.4% of the control group. Death from any cause occurred in 3.1% of the WCD group and 4.9% of the control group. It may be noted that of the 48 participants in the WCD group who died, only 12 were wearing the device at the time of death. This could have led to the lack of statistical significance in the arrhythmic death between the WCD group and control group in the VEST trial on intention to treat analysis, once again highlighting the compliance factor in WCD.
Per-protocol analysis of VEST trial showed that there was a significant reduction in total and arrhythmic mortality, again highlighting the compliance factor. Some of the factors which independently predicted WCD compliance were cardiac arrest during index myocardial infarction, higher creatinine, diabetes, prior heart failure, and number of WCD alarms. Among the factors predicting worse compliance were higher body mass index, prior percutaneous coronary intervention and any WCD shock [8].
2017 AHA/ACC/HRS Guideline had given Class IIa recommendation for the use of WCD in secondary prevention when ICD removal is required as with infection, and IIb recommendation for all other scenarios, including primary prevention [9]. The class IIb indications included patients waiting for a cardiac transplant, post myocardial infarction patients with left ventricular ejection fraction of 35% or less within 40 days of infarction, newly diagnosed non-ischemic cardiomyopathy, revascularization within past 90 days, myocarditis or secondary cardiomyopathy and systemic infection. Basically these were patients at increased risk of sudden cardiac death, but ineligible for an ICD.
A systematic review published in 2021 evaluated the evidence on WCD in patients undergoing ICD explant/lead extraction. They also compared the use and costs of WCD with standard therapy (in-hospital stay). Their cost minimization analysis showed a cost reduction of 1782 Euros per patient using WCD. The calculation was based on a reduction of hospital stay from 21 days to 15 days with the use of WCD. They noted that after ICD explantation, patients can be safely and effectively protected from sudden cardiac death after hospital discharge through WCD utilization [10].
References
- Reek S, Geller JC, Meltendorf U, Wollbrueck A, Szymkiewicz SJ, Klein HU. Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol. 2003 Oct;26(10):2016-22. doi: 10.1046/j.1460-9592.2003.00311.x. PMID: 14516344.
- Francis J, Reek S. Wearable cardioverter defibrillator: a life vest till the life boat (ICD) arrives. Indian Heart J. 2014 Jan-Feb;66(1):68-72. doi: 10.1016/j.ihj.2013.12.050. Epub 2014 Jan 8. PMID: 24581099; PMCID: PMC3946444.
- Naniwadekar A, Alnabelsi T, Joshi K, Obasare E, Greenspan A, Mainigi S. Real world utilization and impact of the wearable cardioverter-defibrillator in a community setting. Indian Pacing Electrophysiol J. 2017 May-Jun;17(3):65-69. doi: 10.1016/j.ipej.2017.01.003. Epub 2017 Jan 9. PMID: 29072998; PMCID: PMC5478916.
- Francis J. How good is your life vest in the real world? Indian Pacing Electrophysiol J. 2017 May-Jun;17(3):63-64. doi: 10.1016/j.ipej.2017.06.001. Epub 2017 Jun 14. PMID: 29072997; PMCID: PMC5478938.
- Dillon KA, Szymkiewicz SJ, Kaib TE. Evaluation of the effectiveness of a wearable cardioverter defibrillator detection algorithm. J Electrocardiol. 2010 Jan-Feb;43(1):63-7. doi: 10.1016/j.jelectrocard.2009.05.010. PMID: 19570548.
- Masri A, Altibi AM, Erqou S, Zmaili MA, Saleh A, Al-Adham R, Ayoub K, Baghal M, Alkukhun L, Barakat AF, Jain S, Saba S, Adelstein E. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol. 2019 Feb;5(2):152-161. doi: 10.1016/j.jacep.2018.11.011. Epub 2019 Jan 30. PMID: 30784684; PMCID: PMC6383782.
- Olgin JE, Pletcher MJ, Vittinghoff E, Wranicz J, Malik R, Morin DP, Zweibel S, Buxton AE, Elayi CS, Chung EH, Rashba E, Borggrefe M, Hue TF, Maguire C, Lin F, Simon JA, Hulley S, Lee BK; VEST Investigators. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N Engl J Med. 2018 Sep 27;379(13):1205-1215. doi: 10.1056/NEJMoa1800781. PMID: 30280654; PMCID: PMC6276371.
- Olgin JE, Lee BK, Vittinghoff E, Morin DP, Zweibel S, Rashba E, Chung EH, Borggrefe M, Hulley S, Lin F, Hue TF, Pletcher MJ. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J Cardiovasc Electrophysiol. 2020 May;31(5):1009-1018. doi: 10.1111/jce.14404. Epub 2020 Mar 3. PMID: 32083365.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018 Oct 2;72(14):e91-e220. doi: 10.1016/j.jacc.2017.10.054. Epub 2018 Aug 16. Erratum in: J Am Coll Cardiol. 2018 Oct 2;72(14):1760. PMID: 29097296.
- Boriani G, Mantovani LG, Cortesi PA, De Ponti R, D’Onofrio A, Arena G, Curnis A, Forleo G, Guerra F, Porcu M, Sgarito G, Botto GL. Cost-minimization analysis of a wearable cardioverter defibrillator in adult patients undergoing ICD explant procedures: Clinical and economic implications. Clin Cardiol. 2021 Nov;44(11):1497-1505. doi: 10.1002/clc.23709. Epub 2021 Aug 24. PMID: 34427926; PMCID: PMC8571546.
