Transcatheter tricuspid valve replacement
Transcatheter tricuspid valve replacement
Transcatheter treatment options for severe symptomatic tricuspid regurgitation is an evolving science. Very often these patients are very sick and having multiple comorbidities increasing their risk for surgical management of tricuspid valve disease. Many have associated ascites and chronic kidney disease.
A case report in JACC Cardiovascular Interventions described a patient with torrential tricuspid regurgitation and NYHA class III symptoms [1]. He had past history of coronary artery bypass graft surgery and atrial fibrillation. Echocardiography showed leaflet tethering and a 15-mm coaptation gap. Mild right ventricular dysfunction and preserved left ventricular function were noted. Computed tomography documented 49 mm diameter for the tricuspid valve.
48-mm EVOQUE valve was implanted using a 28-F EVOQUE system (Edwards Lifesciences, Irvine, California) by a transfemoral venous route. Guidance was by both transesophageal echocardiography and fluoroscopy. There was mild paravalvar leak and a mean gradient of 2 mm Hg after the procedure. He had excellent clinical response and was discharged on anticoagulation. Follow up at 6 months documented NYHA class I functional status, increase in six minute walk distance by 200 meters and 45 point improvement on the Minnesota Living With Heart Failure Questionnaire for assessment of quality of life.
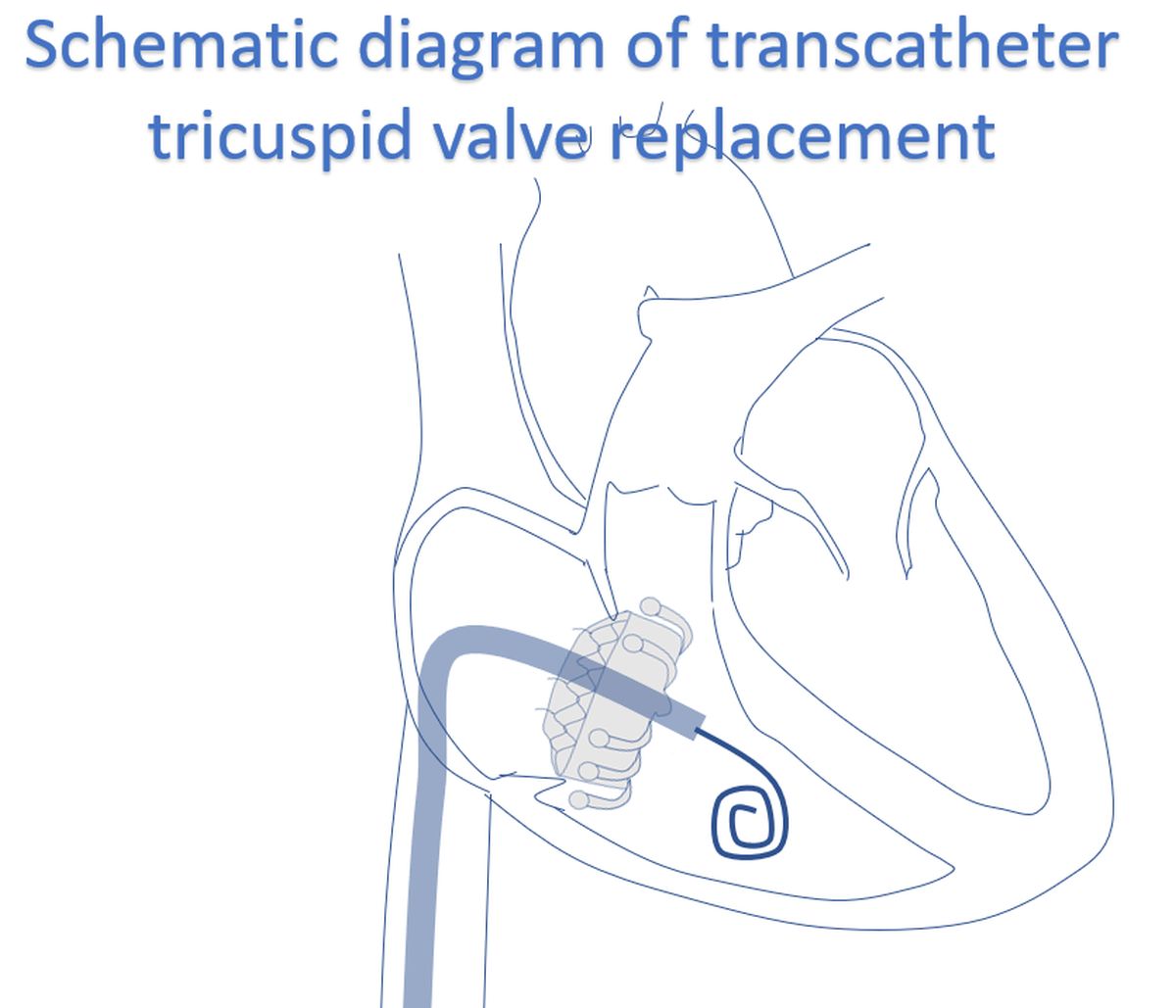
This can be considered as a typical result of transcatheter tricuspid valve replacement by the investigational EVOQUE valve. As you can see, case selection is after transesophageal echocardiography and computed tomography. A multicenter, observational, first-in-human experience was published in 2021 [2]. Twenty-five patients with severe tricuspid regurgitation (predominantly functional) underwent EVOQUE transcatheter tricuspid valve replacement in a compassionate-use experience.
92% technical success was achieved, with no mortality during the procedure or conversion to surgery. 30 day mortality was also zero. 76% of patients were in NYHA functional class I or II at 30 day follow up and the tricuspid regurgitation grade was 2+ or lesser in 96%. Major bleeding was noted in 3 patients, 2 patients required a pacemaker implantation and one patient required dialysis.
Other options which have been tried for tricuspid regurgitation are TriClip (Abbott Vascular, Santa Clara, California) and Pascal (Edwards Lifesciences, Irvine, California). Those were transcatheter edge-to-edge repair devices [3]. TRILUMINATE (Trial to Evaluate Treatment With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater Tricuspid Regurgitation) documented reduction of tricuspid regurgitation severity by at least one grade at 30 days in 86% of the 83 patients who had available echocardiogram data and imaging [4]. In that study, tricuspid regurgitation was graded as mild, moderate, severe, massive, and torrential.
As per the ClinicalTrials.gov Identifier: NCT04221490, TRISCEND Study of the EVOQUE valve was started in 2020 and is expected to be completed in 2025, with estimated enrollment of 200 patients [5]. TRISCEND II Pivotal Trial listed as ClinicalTrials.gov Identifier: NCT04482062 was started in 2021 and is expected to be completed in 2028 [6]. Expected enrollment is 775 participants.
Six-month outcomes of the TRISCEND presented at TCT 2021 mentioned that the favorable 30-day outcomes were sustained at 6 months [7]. 6-month follow-up was available for 56 of the enrolled 132 patients. Baseline tricuspid regurgitation grade was at least severe in 88% and 90% had atrial fibrillation. Two thirds of the patients were in functional class III/IV heart failure prior to device implantation. Procedural success was noted as 96.2% with 94.7% having right femoral access.
At 30 days, there were three cardiovascular deaths, one renal complication, 22 severe bleeds and two major access site and vascular complications. Two had reinterventions and one had major cardiac structural complication. All cause mortality was 3.2% and new permanent pacemaker was required in 8 patients.
Mild tricuspid regurgitation was noted in 49% at 6 months while the remaining had trace or none. Survival rate was 96% and freedom from heart failure hospitalization was 94%. 89% of patients were in functional class I or II. 6 minute walk distance had increased by 56 meters and Kansas City Cardiomyopathy Questionnaire score increased by 27 points. The high bleeding rates are an important concern in this study. Many were not at access site and included urinary tract and gastrointestinal bleeding. Management of anticoagulation in this population with high bleeding risk probably needs modification.
References
- Fam NP, Ong G, Deva DP, Peterson MD. Transfemoral Transcatheter Tricuspid Valve Replacement. JACC Cardiovasc Interv. 2020 May 25;13(10):e93-e94. doi: 10.1016/j.jcin.2020.01.194. Epub 2020 Mar 11. PMID: 32171717.
- Fam NP, von Bardeleben RS, Hensey M, Kodali SK, Smith RL, Hausleiter J, Ong G, Boone R, Ruf T, George I, Szerlip M, Näbauer M, Ali FM, Moss R, Bapat V, Schnitzler K, Kreidel F, Ye J, Deva DP, Mack MJ, Grayburn PA, Peterson MD, Leon MB, Hahn RT, Webb JG. Transfemoral Transcatheter Tricuspid Valve Replacement With the EVOQUE System: A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc Interv. 2021 Mar 8;14(5):501-511. doi: 10.1016/j.jcin.2020.11.045. Epub 2021 Feb 10. PMID: 33582084.
- Dreyfus GD, Essayagh B. Transcatheter Treatment Options for Severe Tricuspid Regurgitation: And the Winner Is Valve Replacement? JACC Cardiovasc Interv. 2021 Mar 8;14(5):512-514. doi: 10.1016/j.jcin.2021.01.012. Epub 2021 Feb 10. PMID: 33582087.
- Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Näbauer M, Dahou A, Hahn RT. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019 Nov 30;394(10213):2002-2011. doi: 10.1016/S0140-6736(19)32600-5. Epub 2019 Nov 7. Erratum in: Lancet. 2020 Mar 14;395(10227):870. PMID: 31708188.
- Edwards EVOQUE Tricuspid Valve Replacement: Investigation of Safety and Clinical Efficacy After Replacement of Tricuspid Valve With Transcatheter Device. ClinicalTrials.gov Identifier: NCT04221490. Available at: https://clinicaltrials.gov/ct2/show/study/NCT04221490. Accessed on 20 February 2022.
- Edwards EVOQUE Transcatheter Tricuspid Valve Replacement: Pivotal Clinical Investigation of Safety and Clinical Efficacy Using a Novel Device. ClinicalTrials.gov Identifier: NCT04482062. Available at: https://clinicaltrials.gov/ct2/show/NCT04482062. Accessed on 20 February 2022.
- TRISCEND at 6 Months. Available at: https://www.tctmd.com/news/transcatheter-mitral-tricuspid-valve-replacement-inch-forward. Accessed on 20 February 2022.

