Rheumatic mitral stenosis
Rheumatic mitral stenosis
Rheumatic mitral stenosis develops a long period after the acute episode of rheumatic fever as it takes a long time for fibrosis and mitral valve narrowing to set in. Commisural fusion is the hallmark of rheumatic mitral stenosis. It associated with a varying degree of fusion of subvalvar apparatus (called ‘subvalvar pathology’). Chordae tendinae are shortened and fibrotic. This often produces a secondary obstruction below the mitral valve. Long standing cases have associated calcification of leaflets and subvalvar pathology. Rheumatic mitral stenosis may take decades to develop in certain cases. The following chronological classification can be considered:
- Juvenile mitral stenosis: Onset before the age of 20 years, the term coined by Prof. SB Roy, seen mostly in developing countries. Hemoptysis, calcification and atrial fibrillation are less common in this group. But they have a more severe course.
- Adult onset mitral stenosis: This was the standard pattern seen in Western world with onset of symptoms of mitral stenosis occurring in the third and fourth decade.
- Late onset mitral stenosis: This is a relatively new group which has been noted in the West with initial manifestation in the elderly.
Pathologically, the mitral valve in mitral stenosis has a classical ‘fish mouth appearance’.
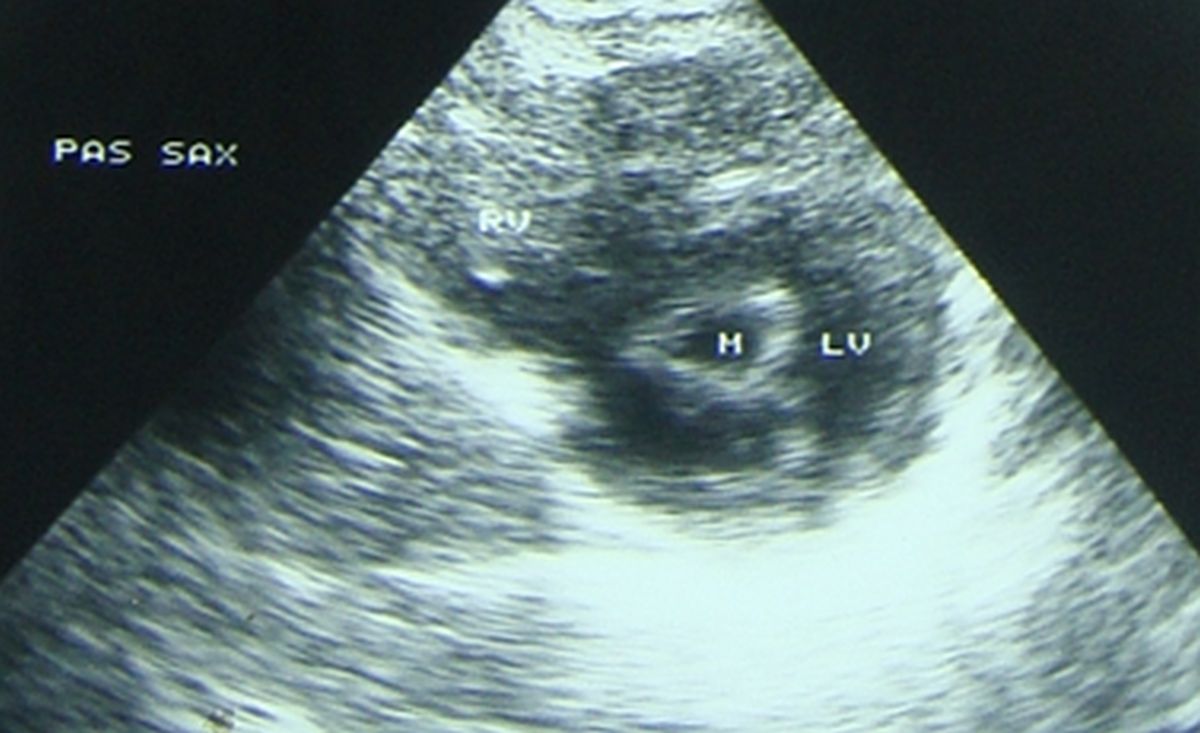
Cross sectional view of the mitral valve on echocardiogram in mitral stenosis (M: mitral orifice) with fish mouth appearance. It can be seen that mitral leaflets are thickened and the commissures are fused. PAS SAX: Parasternal short axis view, RV: Right ventricle, LV: Left ventricle in cross section.
In long standing severe mitral stenosis, left atrium enlarges and pulmonary venous pressure increases. Increase in pulmonary venous pressure is transmitted back to the pulmonary arteries producing pulmonary arterial hypertension. This in turn produces right ventricular hypertrophy. Severe right ventricular hypertrophy and failure leads to tricuspid regurgitation and right atrial enlargement.
The predominant symptom of mitral stenosis is exertional breathlessness due to pulmonary congestion. It may manifest as episodes in the night known as paroxysmal nocturnal dyspnea. Other symptoms are hemoptysis due to rupture of bronchial veins or pulmonary infarction and palpitation. Atrial fibrillation which occurs in chronic cases may contribute to palpitation and precipitation of heart failure.
Clinical findings in mitral stenosis includes a loud first heart sound, opening snap and mid diastolic murmur with presystolic accentuation. Opening snap becomes inaudible when the mitral valve is calcified. Presystolic accentuation is absent when there is atrial fibrillation. Left parasternal heave and loud pulmonary component of second heart sound (P2) can be noted when there is severe pulmonary hypertension. An early diastolic murmur of pulmonary regurgitation (Graham Steell murmur) can also occur in severe pulmonary hypertension. Pansystolic murmur of tricuspid regurgitation may be noted along with this.
In the yester years, severe mitral stenosis used to be treated with closed (CMV) or open mitral valvotomy (OMV). Severely calcific valves are replaced with prosthetic valves (MVR). For the past few decades, catheter based balloon mitral valvotomy (BMV), otherwise known as percutaneous mitral commissurotomy (PTMC) has become the first option for mitral stenosis with pliable valves which do not have dense calcification. Those with atrial fibrillation need anticoagulation as they are highly prone for thromboembolic events.